Methyl tert-butyl ether (MTBE), also known as methyl tert-butyl ether and tert-butyl methyl ether, is an organic compound with a structural formula (CH3)3COCH3. MTBE is a volatile, flammable, and colorless liquid that is sparingly soluble in water. Primarily used as a fuel additive, MTBE is blended into gasoline to increase its octane rating and knock resistance, and reduce unwanted emissions.
MTBE is used as a fuel component in fuel for gasoline engines. It is one of a group of chemicals commonly known as oxygenates because they raise the oxygen content of gasoline.
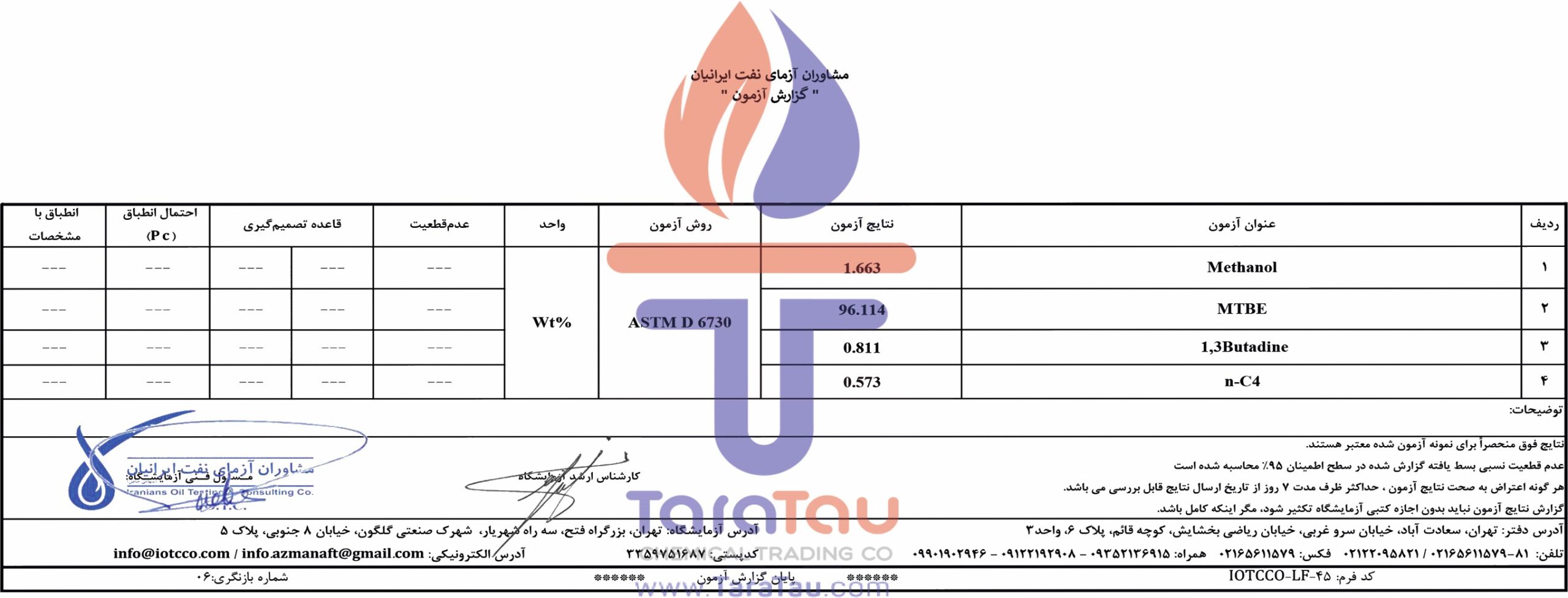
MTBE is manufactured via the chemical reaction of methanol and isobutylene. Methanol is primarily derived from natural gas, where steam reforming converts the various light hydrocarbons in natural gas (primarily methane) into carbon monoxide and hydrogen. The resulting gases then further react in the presence of a catalyst to form methanol. Isobutylene can be produced through a variety of methods. n-butane can be isomerized into isobutane which can be dehydrogenated to isobutylene. In the Halcon process, t-Butyl hydroperoxide derived from isobutane oxygenation is reacted with propylene to produce propylene oxide and t-butanol. The t-butanol can be dehydrated to isobutylene